- HOME >
- For Researchers >
- Product Search >
- Search Result >
- #27751 Human Tenascin-C Large (FNⅢ-C) Assay Kit - IBL
Product Search
#27751 Human Tenascin-C Large (FNⅢ-C) Assay Kit - IBL
- Intended Use:
- Research reagents
- Measuring Method:
- ELISA
- Sample Types:
- Human
- Measuring Samples:
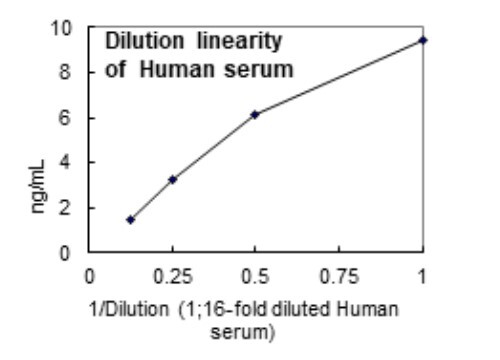
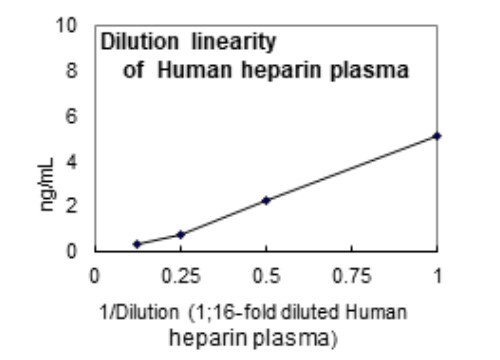
- Heparin-plasma, Serum, Cell culture supernatant
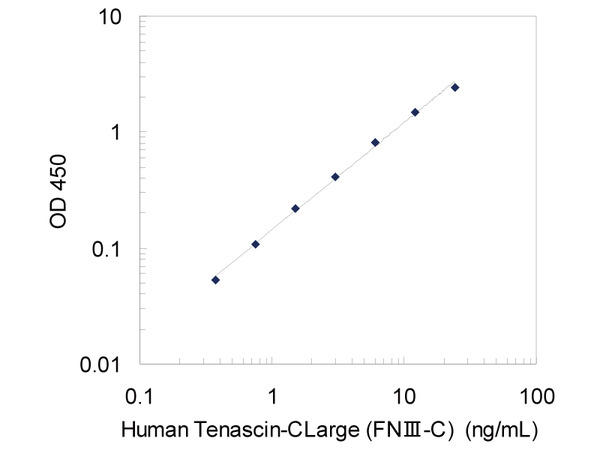
- Measurement Range:
- 0.38 ~ 24 ng/mL
- Package Size1:
- 96 Well
※ The product indicated as "Research reagents" in the column Intended Use cannot be used
for diagnostic nor any medical purpose.
※ The datasheet listed on this page is sample only. Please refer to the datasheet
enclosed in the product purchased before use.
Product Overview
Product Overview
| Product Code | 27751 |
|---|---|
| Product Name | Human Tenascin-C Large (FNⅢ-C) Assay Kit - IBL |
| Maker Name | Immuno-Biological Laboratories Co., Ltd. |
| Intended Use | Research reagents |
| Measuring Method | ELISA |
| Conjugate | HRP |
| Species | Human |
| Measuring Samples | Heparin-plasma, Serum, Cell culture supernatant |
| Measurement Range | 0.38 ~ 24 ng/mL |
| Primary Reaction | 60 minutes at 37 ℃ |
| Secondary Reaction | 30 minutes at 2 - 8 ℃ |
| Sensitivity | 0.01 ng/mL |
| Storage Condition | 2 - 8 ℃ |
| Poisonous and Deleterious Substances | Not Applicable |
| Cartagena | Not Applicable |
| Measuring Service | Not Available |
| Package Size 1 | 96 Well |
Product Description
Product Description
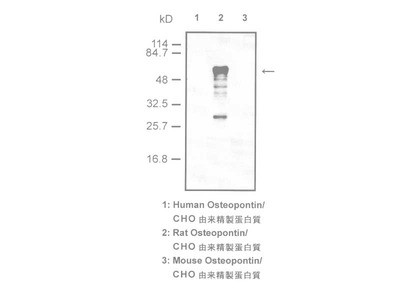
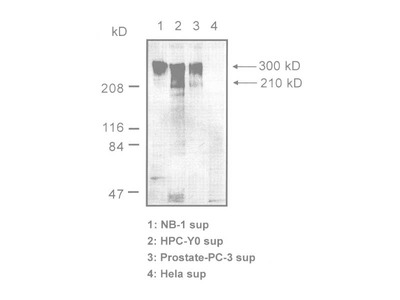
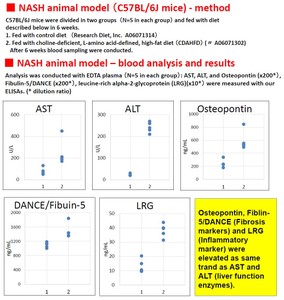
Tenascin-C is an extracellular matrix (ECM) glycoprotein that is composed of 210-400 kDa subunits consisting of four domains. One subunit has a TA domain at the N-terminal end, then an epidermal growth factor-like sequence domain (EGF-like domain), a fibronectin type III(FN III) repeat domain, and a fibrinogen-like domain at the C-terminal end. There is an alternatively spliced domain in the FN III domain, and it generates some types of variants of Tenascin-C. The subunits form a trimer by twisting at the N-terminal coiled domain and form a hexamer by a disulfide bond, in tissue. While low molecular weight variants of Tenascin-C are present in normal tissue, it is said that high molecular variants of Tenascen-C is expressed in various diseased tissue including cancer.
References
References
- Serum tenascin-C predicts resistance to steroid combination therapy in high-risk Kawasaki disease: a multicenter prospective cohort study. Yoshikane Y et al. Multicenter Study Pediatr Rheumatol Online J. 2021 Jun 5;19(1):82.PMID: 34090475
- Serum tenascin-C is independently associated with increased major adverse cardiovascular events and death in individuals with type 2 diabetes: a French prospective cohort. Gellen B et al. Diabetologia. 2020 May;63(5):915-923.PMID: 32040670
- Tenascin C: A Potential Biomarker for Predicting the Severity of Coronary Atherosclerosis. Gao W et al. J Atheroscler Thromb. 2019;26(1):31-38.PMID: 29769455
- Characterization of tenascin-C as a novel biomarker for asthma: utility of tenascin-C in combination with periostin or immunoglobulin E. Yasuda M et al. Allergy Asthma Clin Immunol. 2018 Nov 19;14:72.PMID: 30473714
- Increased Serum Levels of Fetal Tenascin-C Variants in Patients with Pulmonary Hypertension: Novel Biomarkers Reflecting Vascular Remodeling and Right Ventricular Dysfunction? Ilonka Rohm et al. Int J Mol Sci. 2017 Nov 8;18(11):2371.PMID: 29117120
- Serum Tenascin-C as a Novel Predictor for Risk of Coronary Artery Lesion and Resistance to Intravenous Immunoglobulin in Kawasaki Disease - A Multicenter Retrospective Study. Okuma Y et al. Circ J. 2016 Oct 25;80(11):2376-2381PMID: 27746411
- Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Vicens-Zygmunt V et al. Respir Res. 2015 Jul 1;16:82.PMID: 26126411
- Response to infliximab therapy in ulcerative colitis is associated with decreased monocyte activation, reduced CCL2 expression and downregulation of Tenascin C. Magnusson MK et al. J Crohns Colitis. 2015 Jan;9(1):56-65.PMID: 25518051
- Serum Levels of tenascin-C Variants in Congestive Heart Failure Patients: Comparative Analysis of Ischemic, Dilated, and Hypertensive Cardiomyopathy. Marcus Franz et al. Clin Lab. 2014;60(6):1007-13.PMID: 25016707
- Type A dissection and chronic dilatation: tenascin-C as a key factor in destabilization of the aortic wall. Trescher K et al. Interact Cardiovasc Thorac Surg. 2013 Aug;17(2):365-70.PMID: 23656926
- Tenascin-C levels in synovial fluid are elevated after injury to the human and canine joint and correlate with markers of inflammation and matrix degradation. Chockalingam PS et al. Osteoarthritis Cartilage. 2013 Feb;21(2):339-45.PMID: 23142724
- Raised circulating tenascin-C in rheumatoid arthritis. Page TH et al. Arthritis Res Ther. 2012 Nov 29;14(6):R260.PMID: 23193984
- High Circulating Levels of Large Splice Variants of tenascin-C Is Associated With Mortality and Cardiovascular Disease in Chronic Kidney Disease Patients. Liabeuf S et al. Atherosclerosis. 2011 Mar;215(1):116-24.PMID: 21183183
- Incremental prognostic values of serum tenascin-C levels with blood B-type natriuretic peptide testing at discharge in patients with dilated cardiomyopathy and decompensated heart failure. Fujimoto N et al. J Card Fail. 2009 Dec;15(10):898-905.PMID: 19944367
- Higher serum tenascin-C levels reflect the severity of heart failure, left ventricular dysfunction and remodeling in patients with dilated cardiomyopathy. Terasaki F et al. Circ J. 2007 Mar;71(3):327-30.PMID: 17322629
- Changes in biochemical markers and prediction of effectiveness of intra-articular hyaluronan in patients with knee osteoarthritis. Hasegawa M et al. Osteoarthritis Cartilage. 2008 Apr;16(4):526-9. Epub 2007 Oct 22.PMID: 17951079
- Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. Sato A et al. J Am Coll Cardiol. 2006 Jun 6;47(11):2319-25.PMID: 16750702
- Tenascin-C concentration in synovial fluid correlates with radiographic progression of knee osteoarthritis. Hasegawa M et al. J Rheumatol. 2004 Oct;31(10):2021-6.PMID: 15468369
Note: Retrieve by PMID number in displayed by abstract: http://www.ncbi.nlm.nih.gov
FAQ
FAQ
-
 Q.Can this kit be used for mouse or rat samples?
Q.Can this kit be used for mouse or rat samples? -
 A.No, they cannot.
A.No, they cannot. -
 Q.Is composition of EIA buffer of each ELISA kit all same? Can it be mixed to use?
Q.Is composition of EIA buffer of each ELISA kit all same? Can it be mixed to use?
ELISA common FAQ -
 A.No it isn't. As constitute of each EIA buffer is different, it cannot be mixed with other lots or EIA buffers contained in other kind of ELISA kits.
A.No it isn't. As constitute of each EIA buffer is different, it cannot be mixed with other lots or EIA buffers contained in other kind of ELISA kits. -
 Q.What is the composition of concentrated wash buffer?
Q.What is the composition of concentrated wash buffer?
ELISA common FAQ -
 A.It contains ordinary Tween and phosphate buffer (0.05% Tween-20 in PB).
A.It contains ordinary Tween and phosphate buffer (0.05% Tween-20 in PB). -
 Q.What is the feature of the plate?
Q.What is the feature of the plate?
ELISA common FAQ -
 A.We use plate that is flat bottom and removable strip type plate (8wellx 12 strips).
A.We use plate that is flat bottom and removable strip type plate (8wellx 12 strips). -
 Q.Can I re-use standard after reconstitution?
Q.Can I re-use standard after reconstitution?
ELISA common FAQ -
 A.Not recommended to re-use standard after reconstitution. Please use it at once after the reconstitution.
A.Not recommended to re-use standard after reconstitution. Please use it at once after the reconstitution.
Please note that there are some exceptions. One time freeze-thaw the standard is acceptable for use after reconstitution for some ELISAs.
Please check the details on each product datasheet. -
 Q.What is different between reagent blank and test sample blank?
Q.What is different between reagent blank and test sample blank?
ELISA common FAQ -
 A.Reagent blank means a well is only added EIA buffer and the purpose is confirming whether the Test sample value is influenced by lack of washing process or other operations. Test sample blank means a well is added EIA buffer and HRP antibody and the purpose is to calculate the background.
A.Reagent blank means a well is only added EIA buffer and the purpose is confirming whether the Test sample value is influenced by lack of washing process or other operations. Test sample blank means a well is added EIA buffer and HRP antibody and the purpose is to calculate the background. -
 Q.How many samples can be measured by this kit?
Q.How many samples can be measured by this kit?
ELISA common FAQ -
 A.The pre-coated plate contained in our ELISA kit is 96 wells plate. We recommend to use 16 wells (2 slits) for standard and 80 wells (10 slits) for 40 samples in duplicate.
A.The pre-coated plate contained in our ELISA kit is 96 wells plate. We recommend to use 16 wells (2 slits) for standard and 80 wells (10 slits) for 40 samples in duplicate. -
 Q.What is LOD (Limit of Detection)?
Q.What is LOD (Limit of Detection)?
ELISA common FAQ -
 A.It (LOD) is defined as sensitivity that is calculated using the NCCSL method. Please refer to a datasheet of each product.
A.It (LOD) is defined as sensitivity that is calculated using the NCCSL method. Please refer to a datasheet of each product. -
 Q.What is LOQ (Limit of Quantification)?
Q.What is LOQ (Limit of Quantification)?
ELISA common FAQ -
 A.It (LOQ) is the lowest value of measurement (standard) range. Please refer to a datasheet of each product.
A.It (LOQ) is the lowest value of measurement (standard) range. Please refer to a datasheet of each product. -
 Q.What is the definition of Over Night (O/N) reaction?
Q.What is the definition of Over Night (O/N) reaction?
ELISA common FAQ -
 A.It means that the reaction is required more than 16 hours unless otherwise specifically defined it on a datasheet of each ELISA product.
A.It means that the reaction is required more than 16 hours unless otherwise specifically defined it on a datasheet of each ELISA product. -
 Q.What is the specification of quality control for ELISA product release?
Q.What is the specification of quality control for ELISA product release?
ELISA common FAQ -
 A.The information of specification is available on individual lot specific CoA. Please contact us with your reference lot number for obtaining of specific CoA.
A.The information of specification is available on individual lot specific CoA. Please contact us with your reference lot number for obtaining of specific CoA. -
 Q.What is the number (e.g. 432143214321) at the edge of strips of the plate?
Q.What is the number (e.g. 432143214321) at the edge of strips of the plate?
ELISA common FAQ -
 A.According to the plate maker (ThermoFisher), it does not have any specific meaning as it is just the number of molds.
A.According to the plate maker (ThermoFisher), it does not have any specific meaning as it is just the number of molds. -
 Q.How to wash an ELISA plate?
Q.How to wash an ELISA plate?
ELISA common FAQ -
 A.Washing it by an auto-washer is highly recommended.
A.Washing it by an auto-washer is highly recommended.
If it is not available, please refer to the demo video (only 2 mins) using a washing bottle. -
 Q.The wells turned black during the test with the kit.
Q.The wells turned black during the test with the kit.
ELISA common FAQ -
 A.It is possible that the wells were not washed sufficiently during the washing process after the HRP-labeled antibody reaction.
A.It is possible that the wells were not washed sufficiently during the washing process after the HRP-labeled antibody reaction.
Be sure to wash the wells enough times as described in the data sheet with washing buffer of more than 350 µL.