- HOME >
- For Researchers >
- Product Search >
- Search Result >
- #27900 Human Titin N-Fragment (Urine) ELISA Kit – IBL
Product Search
#27900 Human Titin N-Fragment (Urine) ELISA Kit – IBL
- Intended Use:
- Research reagents
- Measuring Method:
- ELISA
- Sample Types:
- Human
- Measuring Samples:
- Urine
- Measurement Range:
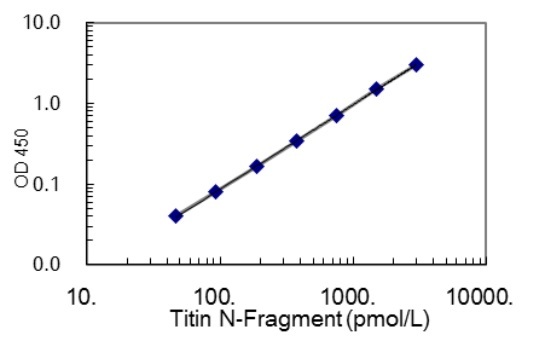
- 46.88 ~ 3000 pmol/L
- Package Size1:
- 96 Well
※ The product indicated as "Research reagents" in the column Intended Use cannot be used
for diagnostic nor any medical purpose.
※ The datasheet listed on this page is sample only. Please refer to the datasheet
enclosed in the product purchased before use.
Product Overview
Product Overview
| Product Code | 27900 |
|---|---|
| Product Name | Human Titin N-Fragment (Urine) ELISA Kit – IBL |
| Maker Name | Immuno-Biological Laboratories Co., Ltd. |
| Intended Use | Research reagents |
| Measuring Method | ELISA |
| Conjugate | HRP |
| Species | Human |
| Measuring Samples | Urine |
| Measurement Range | 46.88 ~ 3000 pmol/L |
| Primary Reaction | 60 minutes at 37°C |
| Secondary Reaction | 30 minutes at 37℃ |
| Sensitivity | 27.91 pmol/L |
| Storage Condition | 2~8°C |
| Poisonous and Deleterious Substances | Not Applicable |
| Cartagena | Not Applicable |
| Measuring Service | Available |
| Package Size 1 | 96 Well |
Product Description
Product Description
Titin (connectin) is a protein that consists of 34,350 amino-acid and specifically expresses in a cross-striated muscle. The molecular weight of human titin is 3,816 kDa and it has been known that as the largest protein among of existing proteins in a living body.
It is one of sarcomere structured protein that is a minimum unit of myofibrillary protein and it has a role as an elastic protein for recovering the length of shortened sarcomere by its contraction. It has been known that titin is cleaved by protein metabolic enzymes such as calpain and matrix metalloprotease if muscles are damaged.
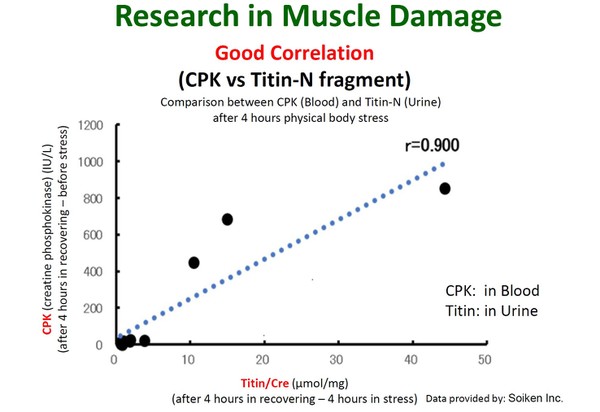
It has been researched in the field of sports medicine, cardiac disease, NAFLD, sarcopenia and frailty and it is also considered that it can be a candidate as a non-invasive biomarker for monitoring status of muscles. This ELISA kit can measure Titin N fragment in human urine.
The control set is available for sale on request. Learn More
【Topics】
It was reported that urinary titin can be a non-invasive biomarker for Duchenne muscular dystrophy (DMD).
Learn More >External News Link >Paper Link
It is one of sarcomere structured protein that is a minimum unit of myofibrillary protein and it has a role as an elastic protein for recovering the length of shortened sarcomere by its contraction. It has been known that titin is cleaved by protein metabolic enzymes such as calpain and matrix metalloprotease if muscles are damaged.
It has been researched in the field of sports medicine, cardiac disease, NAFLD, sarcopenia and frailty and it is also considered that it can be a candidate as a non-invasive biomarker for monitoring status of muscles. This ELISA kit can measure Titin N fragment in human urine.
The control set is available for sale on request. Learn More
【Topics】
It was reported that urinary titin can be a non-invasive biomarker for Duchenne muscular dystrophy (DMD).
Learn More >External News Link >Paper Link
References
References
- Urinary Titin Level Is a Novel Marker of Severe Sarcopenia and Dynapenia: Shimane CoHRE Study. Kanako Hara et al. JCSM Communications, 2025; 8:e70006 1-10PMID:
- The N-Terminal Fragment of Urine Titin Is Not a Product of Degradation by Calpain 3. Nambu Y et al. Muscle Nerve. 2025 Jan 8.PMID: 39777416
- The Effect of Prior Creatine Intake for 28 Days on Accelerated Recovery from Exercise-Induced Muscle Damage: A Double-Blind, Randomized, Placebo-Controlled Trial. Yamaguchi S et al. Nutrients. 2024 Mar 20;16(6):896.PMID: 38542807
- N-terminal titin fragment: a non-invasive, pharmacodynamic biomarker for microdystrophin efficacy. Boehler JF et al. Skelet Muscle. 2024 Jan 16;14(1):2.PMID: 38229112
- The Utility of Urinary Titin to Diagnose and Predict the Prognosis of Acute Myocardial Infarction. Arase M et al. Int J Mol Sci. 2024 Jan 1;25(1):573.PMID: 38203744
- Relationships between Changes in Muscle Shear Modulus, Urinary Titin N- Terminal Fragment, and Maximum Voluntary Contraction Torque after Eccentric Exercise of the Elbow Flexors. Inami T et al. J Sports Sci Med. 2023 Dec 1;22(4):797-805.PMID: 38045754
- Urinary titin N-fragment as a predictor of decreased skeletal muscle mass in patients with interstitial lung diseases. Hanada M et al. Sci Rep. 2023 Jun 15;13(1):9723.PMID: 37322176
- Urinary titin in myotonic dystrophy type 1. Varga D et al. Muscle Nerve. 2023 Jun 8.PMID: 37291994
- Urine titin as a novel biomarker for Duchenne muscular dystrophy. Ishii MN et al. Neuromuscul Disord. 2023 Feb 13;33(4):302-308.PMID: 36871413
- Coagulopathy correlates with muscle titin injury in critically ill patients. Nakamura K et al. J Crit Care. 2022 Dec 22;74:154234.PMID: 36565648
- Changes in Muscle Shear Modulus and Urinary Titin N-Terminal Fragment after Eccentric Exercise. Inami T et al. J Sports Sci Med. 2022 Dec 1;21(4):536-544.PMID: 36523897
- Novel protocol combining physical and nutrition therapies, Intensive Goal-directed REhabilitation with Electrical muscle stimulation and Nutrition (IGREEN) care bundle. Nakano H et al. Crit Care. 2021 Dec 4;25(1):415.PMID: 34863251
- Assessment of catabolic state in infants with the use of urinary titin N-fragment. Fukushima S et al. Pediatr Res. 2021 Jul 17.PMID: 34274960
- Urinary Titin N-Fragment Evaluation in a Randomized Controlled Trial of Beta-Hydroxy-Beta-Methylbutyrate for Acute Mild Trauma in Older Adults. Nakano H et al. Nutrients. 2021 Mar 10;13(3):899.PMID: 33802012
- Ratio of urinary N-terminal titin fragment to urinary creatinine is a novel biomarker for amyotrophic lateral sclerosis. Yamada S et al. J Neurol Neurosurg Psychiatry. 2021 Mar 18;jnnp-2020-324615.PMID: 33737450
- Urinary N-terminal fragment of titin: A surrogate marker of serum creatine kinase activity after exercise-induced severe muscle damage. Tanabe Y et al. J Sports Sci. 2021 Mar 16;1-8.PMID: 33722155
- Urinary Titin N-Fragment as a Biomarker of Muscle Atrophy, Intensive Care Unit-Acquired Weakness, and Possible Application for Post-Intensive Care Syndrome. Nakanishi N et al. J Clin Med. 2021 Feb 6;10(4):614.PMID: 33561946
- Urinary N-Terminal Fragment of Titin Reflects Muscle Damage After a Soccer Match in Male Collegiate Soccer Players. Tanabe Y et al. J Strength Cond Res. 2021 Feb 1;35(2):360-365.PMID: 33337691
- Urinary titin as a biomarker in Fukuyama congenital muscular dystrophy. Sato T et al. Neuromuscul Disord. 2021 Jan 13;S0960-8966(21)00006-7.PMID: 33563515
- Urinary titin as a biomarker of sarcopenia in diabetes: a propensity score matching analysis. Takiguchi Y et al. J Endocrinol Invest. 2024 Nov 16.PMID: 39549212
- Elevated Urinary Titin and its Associated Clinical Outcomes after Acute Stroke. Ishihara M et al. J Stroke Cerebrovasc Dis. 2020 Dec 23;30(3):105561.PMID: 33360523
- Urinary titin N-terminal fragment concentration is an indicator of preoperative sarcopenia and nutritional status in patients with gastrointestinal tract and hepatobiliary pancreatic malignancies. Miyoshi K et al. Nutrition. Nov-Dec 2020;79-80:110957.PMID: 32866763
- Urine Titin N-fragment as a Biomarker of Muscle Injury for Critical Illness Myopathy. Nakano H et al. Am J Respir Crit Care Med. 2020 Oct 8.PMID: 33030965
- Cellular senescence-mediated exacerbation of Duchenne muscular dystrophy. Sugihara H et al. Sci Rep. 2020 Oct 12;10(1):16385.PMID: 33046751
- Pathological evaluation of rats carrying in-frame mutations in the dystrophin gene: a new model of Becker muscular dystrophy. Teramoto N et al. Dis Model Mech. 2020 Sep 28;13(9):dmm044701.PMID: 32859695
- Urinary Titin Is a Novel Biomarker for Muscle Atrophy in Nonsurgical Critically Ill Patients: A Two-Center, Prospective Observational Study. Nakanishi N et al. Crit Care Med. 2020 Sep;48(9):1327-1333.PMID: 32706557
- Early Initiation of Awake Veno-Venous Extracorporeal Membrane Oxygenation Can Attenuate Muscle Atrophy and Weakness in Acute Respiratory Distress Syndrome. Nakanishi N et al. Cureus. 2020 Aug 21;12(8):e9926.PMID: 32968587
- Urine TITIN N-fragment as a Novel Biomarker for Critical Illness Myopathy: A Pilot Study. Hidehiko N et al. Crit Care. 2020 Apr 28;24(1):177.PMID: 32345334
- Changes in Urinary Titin N-terminal Fragment Concentration after Concentric and Eccentric Exercise. Yamaguchi S et al. J Sports Sci Med. 2020 Feb 24;19(1):121-129. eCollection 2020 Mar.PMID: 32132835
- Changes in urinary titin N-terminal fragments as a biomarker of exercise-induced muscle damage in the repeated bout effect. Yamaguchi S et al. J Sci Med Sport. 2019 Dec 23. pii: S1440-2440(19)31246-0.PMID: 31928880
- Urinary Levels of Titin-N Fragment, a Skeletal Muscle Damage Marker, are Increased in Subjects with Nonalcoholic Fatty Liver Disease. Oshida N et al. Sci Rep. 2019 Dec 20;9(1):19498.PMID: 31862937
- Titin in muscular dystrophy and cardiomyopathy: Urinary titin as a novel marker. Misaka T et al. Clin Chim Acta. 2019 Aug;495:123-128.PMID: 30959043
- Urinary Titin Is Increased in Patients After Cardiac Surgery. Tanihata J et al. Front Cardiovasc Med. 2019 Feb 8;6:7.PMID: 30800662
- Titin fragment in urine: A noninvasive biomarker of muscle degradation. Matsuo M et al. Adv Clin Chem. 2019;90:1-23.PMID: 31122607
- Receiver operating curve analyses of urinary titin of healthy 3-y-old children may be a noninvasive screening method for Duchenne muscular dystrophy. Matsuo M et al. Clin Chim Acta. 2018 Nov;486:110-114.PMID: 30053403
- Urinary N-terminal fragment of titin is a marker to diagnose muscular dystrophy in patients with cardiomyopathy. Yoshihisa A et al. Clin Chim Acta. 2018 Sep;484:226-230.PMID: 29870683
- Usefulness of Urinary N-Terminal Fragment of Titin to Predict Mortality in Dilated Cardiomyopathy. Yoshihisa A et al. Am J Cardiol. 2018 Feb 12. pii: S0002-9149(18)30192-9.PMID: 29523227
- Diagnostic and clinical significance of the titin fragment in urine of Duchenne muscular dystrophy patients. Awano H et al. Clin Chim Acta. 2018 Jan;476:111-116.PMID: 29175173
- Establishment of a highly sensitive sandwich ELISA for the N-terminal fragment of titin in urine. Maruyama N et al. Sci Rep. 2016 Dec 19;6:39375PMID: 27991570
Note: Retrieve by PMID number in displayed by abstract: http://www.ncbi.nlm.nih.gov
FAQ
FAQ
-
 Q.If urine samples are not measured immediately after collection, how should they be stored?
Q.If urine samples are not measured immediately after collection, how should they be stored? -
 A.They should be store at -20°C or below immediately after collection.
A.They should be store at -20°C or below immediately after collection. -
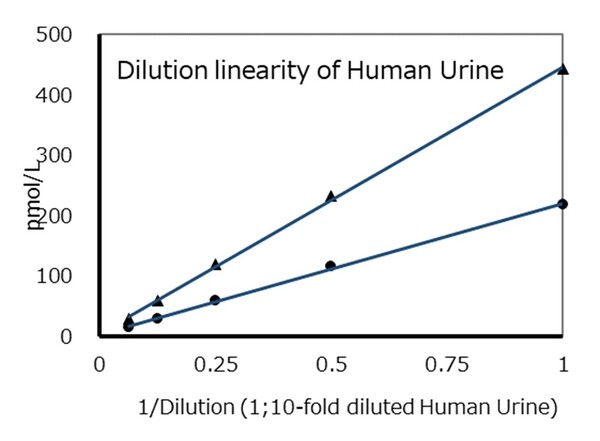
 Q.Is any normalization needed for measured value of urine sample?
Q.Is any normalization needed for measured value of urine sample? -
 A.Yes, it is needed.
A.Yes, it is needed.
Please measure urinary creatinine at the same time and normalize to it. -
 Q.Can rodent (mouse or rat) sample be used for this kit?
Q.Can rodent (mouse or rat) sample be used for this kit? -
 A.No, it cannot.
A.No, it cannot. -
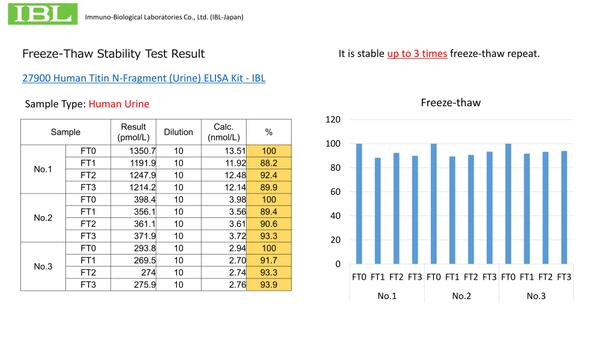
 Q.How many time freeze-thaw can be repeated for human urine sample for the assay?
Q.How many time freeze-thaw can be repeated for human urine sample for the assay? -
 A.It is stable up to 3 times freeze-thaw repeat. Please refer to the freeze-thaw stability test result from this link.
A.It is stable up to 3 times freeze-thaw repeat. Please refer to the freeze-thaw stability test result from this link. -
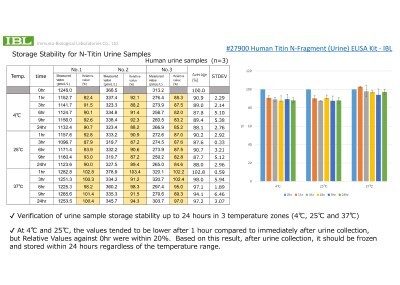
 Q.Do you have any storage (temperature) stability data of urinary titin N-terminal fragment (UTF)?
Q.Do you have any storage (temperature) stability data of urinary titin N-terminal fragment (UTF)? -
 A.Yes, we do. Please refer to the data.
A.Yes, we do. Please refer to the data. -
 Q.Is composition of EIA buffer of each ELISA kit all same? Can it be mixed to use?
Q.Is composition of EIA buffer of each ELISA kit all same? Can it be mixed to use?
ELISA common FAQ -
 A.No it isn't. As constitute of each EIA buffer is different, it cannot be mixed with other lots or EIA buffers contained in other kind of ELISA kits.
A.No it isn't. As constitute of each EIA buffer is different, it cannot be mixed with other lots or EIA buffers contained in other kind of ELISA kits. -
 Q.What is the composition of concentrated wash buffer?
Q.What is the composition of concentrated wash buffer?
ELISA common FAQ -
 A.It contains ordinary Tween and phosphate buffer (0.05% Tween-20 in PB).
A.It contains ordinary Tween and phosphate buffer (0.05% Tween-20 in PB). -
 Q.What is the feature of the plate?
Q.What is the feature of the plate?
ELISA common FAQ -
 A.We use plate that is flat bottom and removable strip type plate (8wellx 12 strips).
A.We use plate that is flat bottom and removable strip type plate (8wellx 12 strips). -
 Q.Can I re-use standard after reconstitution?
Q.Can I re-use standard after reconstitution?
ELISA common FAQ -
 A.Not recommended to re-use standard after reconstitution. Please use it at once after the reconstitution.
A.Not recommended to re-use standard after reconstitution. Please use it at once after the reconstitution.
Please note that there are some exceptions. One time freeze-thaw the standard is acceptable for use after reconstitution for some ELISAs.
Please check the details on each product datasheet. -
 Q.What is different between reagent blank and test sample blank?
Q.What is different between reagent blank and test sample blank?
ELISA common FAQ -
 A.Reagent blank means a well is only added EIA buffer and the purpose is confirming whether the Test sample value is influenced by lack of washing process or other operations. Test sample blank means a well is added EIA buffer and HRP antibody and the purpose is to calculate the background.
A.Reagent blank means a well is only added EIA buffer and the purpose is confirming whether the Test sample value is influenced by lack of washing process or other operations. Test sample blank means a well is added EIA buffer and HRP antibody and the purpose is to calculate the background. -
 Q.How many samples can be measured by this kit?
Q.How many samples can be measured by this kit?
ELISA common FAQ -
 A.The pre-coated plate contained in our ELISA kit is 96 wells plate. We recommend to use 16 wells (2 slits) for standard and 80 wells (10 slits) for 40 samples in duplicate.
A.The pre-coated plate contained in our ELISA kit is 96 wells plate. We recommend to use 16 wells (2 slits) for standard and 80 wells (10 slits) for 40 samples in duplicate. -
 Q.What is LOD (Limit of Detection)?
Q.What is LOD (Limit of Detection)?
ELISA common FAQ -
 A.It (LOD) is defined as sensitivity that is calculated using the NCCSL method. Please refer to a datasheet of each product.
A.It (LOD) is defined as sensitivity that is calculated using the NCCSL method. Please refer to a datasheet of each product. -
 Q.What is LOQ (Limit of Quantification)?
Q.What is LOQ (Limit of Quantification)?
ELISA common FAQ -
 A.It (LOQ) is the lowest value of measurement (standard) range. Please refer to a datasheet of each product.
A.It (LOQ) is the lowest value of measurement (standard) range. Please refer to a datasheet of each product. -
 Q.What is the definition of Over Night (O/N) reaction?
Q.What is the definition of Over Night (O/N) reaction?
ELISA common FAQ -
 A.It means that the reaction is required more than 16 hours unless otherwise specifically defined it on a datasheet of each ELISA product.
A.It means that the reaction is required more than 16 hours unless otherwise specifically defined it on a datasheet of each ELISA product. -
 Q.What is the specification of quality control for ELISA product release?
Q.What is the specification of quality control for ELISA product release?
ELISA common FAQ -
 A.The information of specification is available on individual lot specific CoA. Please contact us with your reference lot number for obtaining of specific CoA.
A.The information of specification is available on individual lot specific CoA. Please contact us with your reference lot number for obtaining of specific CoA. -
 Q.What is the number (e.g. 432143214321) at the edge of strips of the plate?
Q.What is the number (e.g. 432143214321) at the edge of strips of the plate?
ELISA common FAQ -
 A.According to the plate maker (ThermoFisher), it does not have any specific meaning as it is just the number of molds.
A.According to the plate maker (ThermoFisher), it does not have any specific meaning as it is just the number of molds. -
 Q.How to wash an ELISA plate?
Q.How to wash an ELISA plate?
ELISA common FAQ -
 A.Washing it by an auto-washer is highly recommended.
A.Washing it by an auto-washer is highly recommended.
If it is not available, please refer to the demo video (only 2 mins) using a washing bottle. -
 Q.The wells turned black during the test with the kit.
Q.The wells turned black during the test with the kit.
ELISA common FAQ -
 A.It is possible that the wells were not washed sufficiently during the washing process after the HRP-labeled antibody reaction.
A.It is possible that the wells were not washed sufficiently during the washing process after the HRP-labeled antibody reaction.
Be sure to wash the wells enough times as described in the data sheet with washing buffer of more than 350 µL. -
 Q.How do you define the acceptance range of quality control of control sets?
Q.How do you define the acceptance range of quality control of control sets?
Control Set Common FAQ -
 A.In principal, it is defined as ±15%-20% based the standard value described on each datasheet.
A.In principal, it is defined as ±15%-20% based the standard value described on each datasheet. -
 Q.What is the shelf life of control set?
Q.What is the shelf life of control set?
Control Set Common FAQ -
 A.The shelf life is 12 months upon the goods is dispatched from our lab if the goods is unopned.
A.The shelf life is 12 months upon the goods is dispatched from our lab if the goods is unopned. -
 Q.What is the storage condition of control sets?
Q.What is the storage condition of control sets?
Control Set Common FAQ -
 A.It should be stored under chilled condition (2~10℃).
A.It should be stored under chilled condition (2~10℃). -
 Q.Can the control set be used after reconstitution?
Q.Can the control set be used after reconstitution?
Control Set Common FAQ -
 A.No it cannot be re-used after the reconstitution.
A.No it cannot be re-used after the reconstitution. -
 Q.What is the package form of control sets?
Q.What is the package form of control sets?
Control Set Common FAQ -
 A.It is lyophilized form.
A.It is lyophilized form.