- HOME >
- For Researchers >
- Product Search >
- Search Result >
- #10379 Anti-Human Amyloidβ E22P (11A1) Mouse IgG MoAb
Product Search
#10379 Anti-Human Amyloidβ E22P (11A1) Mouse IgG MoAb
- Intended Use:
- Research reagents
- Application:
- WB, IP, IHC
- Package Size1:
- 50μg
- Package Size2:
- 5μg
- Note on Application Abbreviations
- WB:Western Blotting
- IP:Immunoprecipitation
- IHC:Immunohistochemistry
※ The product indicated as "Research reagents" in the column Intended Use cannot be used
for diagnostic nor any medical purpose.
※ The datasheet listed on this page is sample only. Please refer to the datasheet
enclosed in the product purchased before use.
Product Overview
Product Overview
| Product Code | 10379 |
|---|---|
| Product Name | Anti-Human Amyloidβ E22P (11A1) Mouse IgG MoAb |
| Intended Use | Research reagents |
| Application | WB, IP, IHC |
| Species | Human |
| Immunizing antigen | Synthetic peptide of E22P- Amyloidβ10-35 part |
| Source | Mouse-Mouse hybridoma (X63 - Ag 8.653 × BALB/c mouse spleen cells) |
| Clone Name | 11A1 |
| Subclass | IgG1 |
| Purification Method | Protein A purified |
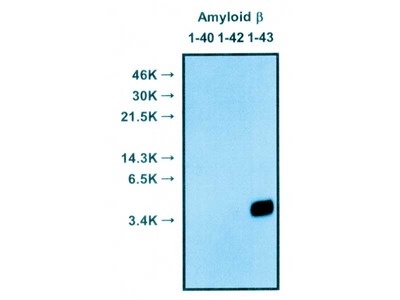
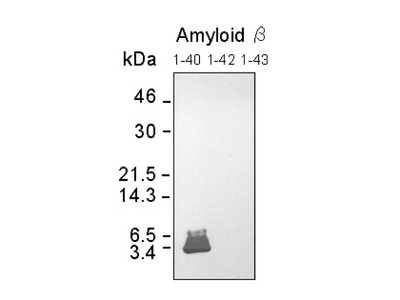
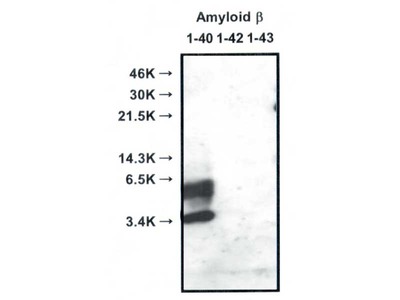
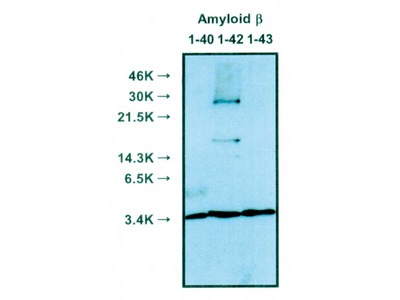
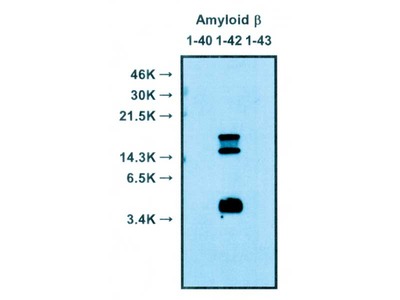
| Specificity | Reacts with native human Amyloidβ 1-40, 1-42 |
| Package Form | Lyophilized product from PBS containing 1 % BSA and 0.05 % NaN3 |
| Storage Condition | 2 - 8℃ |
| Poisonous and Deleterious Substances | Applicable |
| Cartagena | Not Applicable |
| Package Size 1 | 50μg |
| Package Size 2 | 5μg |
| Remarks1 | The commercial use of products without our permission is prohibited. Please make sure to contact us and obtain permission. |
Product Description
Product Description
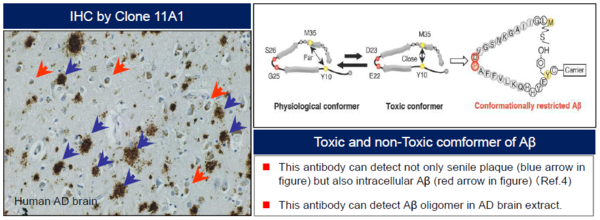
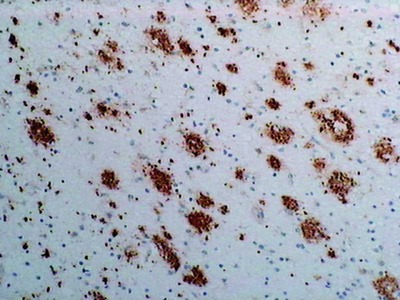
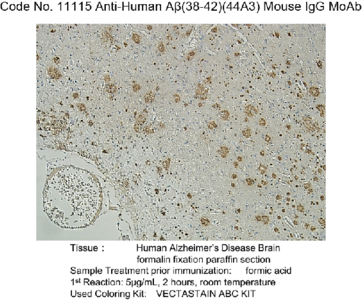
Alzheimer’s disease (AD) is characterized by the presence of extracellular plaques and intracellular neurofibrillary tangles (NFTs) in the brain. Aggregation of the 42-mer amyloid β-protein (Aβ42) plays a critical role in the pathogenesis of AD. Shirasawa and Irie et. al have proposed a toxic conformer with a turn at positions 22 and 23, as well as a nontoxic conformer with a turn at positions 25 and 26, in Aβ42 aggregates from systematic proline scanning and solid-state NMR studies. This monoclonal antibody named 11A1 was developed for toxic Aβ42, using E22P-Aβ10-35, a minimum moiety for neurotoxicity containing the turn at positions 22 and 23, for the generation. Immunohistochemical studies showed that not only extracellular but intracellular amyloid was stained in human AD brains, which suggest that 11A1 could detect toxic oligomers of Aβ with the turn at positions 22 and 23.
References
References
- New Diagnostic Method for Alzheimer's Disease Based on the Toxic Conformation Theory of Amyloid β. Irie K. Biosci Biotechnol Biochem. 2020 Jan;84(1):1-16.PMID: 31538538
- Insulin deficiency promotes formation of toxic amyloid-β42 conformer co-aggregating with hyper-phosphorylated tau oligomer in an Alzheimer's disease model. Imamura T et al. Neurobiol Dis. 2020 Jan 9:104739.PMID: 31927145
- Amyloid β toxic conformer has dynamic localization in the human inferior parietal cortex in absence of amyloid plaques. Kageyama Y et al. Sci Rep. 2018 Nov 15;8(1):16895.PMID: 30442978
- Intracellular amyloid β oligomers impair organelle transport and induce dendritic spine loss in primary neurons. Umeda T et al. Acta Neuropathol Commun. 2015 Aug 21;3:51.PMID: 26293809
- Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice. Umeda T et al. Acta Neuropathol. 2014 May;127(5):685-98.PMID: 24531886
- Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Kondo T et al. Cell Stem Cell. 2013 Apr 4;12(4):487-96.PMID: 23434393
- Early accumulation of intracellular fibrillar oligomers and late congophilic amyloid angiopathy in mice expressing the Osaka intra-Aβ APP mutation. Kulic L et al. Transl Psychiatry. 2012 Nov 13;2(11):e183.PMID: 23149447
- Toxicity in rat primary neurons through the cellular oxidative stress induced by the turn formation at positions 22 and 23 of Aβ42. Izuo N et al. ACS Chem Neurosci. 2012 Sep 19;3(9):674-81.PMID: 23019494/
- Monoclonal antibody against the turn of the 42-residue amyloid β-protein at positions 22 and 23. Murakami K et al. ACS Chem Neurosci. 2010 Nov 17;1(11):747-56.PMID: 22778811
Note: Retrieve by PMID number in displayed by abstract: http://www.ncbi.nlm.nih.gov